When Is It Going to Get Cold Again 2017
T he cold has the twin distinction of being both the world's most widespread infectious disease and one of the most elusive. The name is a trouble, for starters. In nigh every Indo-European language, ane of the words for the affliction relates to low temperature, yet experiments have shown that low temperature neither increases the likelihood of catching a cold, nor the severity of symptoms. Then there is the "common" role, which seems to imply that there is a single, indiscriminate pathogen at large. In reality, more than than 200 viruses provoke cold-like illness, each one deploying its own peculiar chemic and genetic strategy to evade the body'south defences.
It is difficult to think of some other disease that inspires the same level of collective resignation. The common common cold slinks through homes and schools, towns and cities, making people miserable for a few days without warranting much afterthought. Adults endure an average of between two and four colds each yr, and children up to 10, and nosotros have come up to accept this equally an inevitable part of life.
Public understanding remains a jumble of folklore and false assumption. In 1984, researchers at the Academy of Wisconsin-Madison decided to investigate ane of the all-time-known ways of catching a cold. They infected volunteers with a cold virus and instructed them to kiss good for you examination subjects on the oral cavity for at least one minute. (The instruction for participants was to utilise whichever technique was "well-nigh natural".) 16 salubrious volunteers were kissed past people with colds. The outcome: merely 1 confirmed infection.
The most common behavior about how to care for the illness take turned out to exist faux. Dubious efficacy has done little to deter humankind from formulating remedies. The Ebers Papyrus, a medical document from aboriginal Egypt dated to 1550BC, advises a cold sufferer to recite an incantation, "in association with the administration of milk of one who has borne a male child, and fragrant gum". In 1924, US President Calvin Coolidge sat downward in an closed chlorine chamber and inhaled the pungent, baneful gas for almost an hour on the advice of his physicians, who were certain that his cold would be cured quickly. (Information technology wasn't.)
Today, "winter remedy" sales in the Uk reach £300m each year, though most over-the-counter products have non actually been proven to work. Some contain paracetamol, an constructive analgesic, just the dosage is frequently sub-optimal. Taking vitamin C in regular doses does little to ward off affliction. Hot toddies, medicated tissues and allowed organisation "boosts" of echinacea or ginger are ineffective. Antibiotics exercise nix for colds. The only failsafe means of avoiding a common cold is to live in complete isolation from the rest of humanity.
Although modern science has changed the way medicine is practised in almost every field, it has so far failed to produce whatever radically new treatments for colds. The difficulty is that while all colds experience much the aforementioned, from a biological perspective the only common feature of the diverse viruses that crusade colds is that they have adjusted to enter and damage the cells that line the respiratory tract. Otherwise, they belong to quite different categories of organisms, each with a distinct way of infecting our cells. This makes a take hold of-all treatment extremely tricky to formulate.
Scientists today identify vii virus families that cause the majority of colds: rhinovirus, coronavirus, influenza and parainfluenza virus, adenovirus, respiratory syncytial virus (RSV) and, finally, metapneumovirus, which was commencement isolated in 2001. Each has a branch of sub-viruses, known equally serotypes, of which there are nigh 200. Rhinovirus, the smallest cold pathogen by size, is by far the nigh prevalent, causing upwards to three-quarters of colds in adults. To crush the common cold we will need to tackle all of these different families of virus at some stage. But, for at present, rhinovirus is the biggest player.
Scientists first attempted to make a rhinovirus vaccine in the 1950s. They used a reliable method, pioneered past French biologist Louis Pasteur in the 1880s, in which a small amount of virus is introduced to a host in social club to provoke a defensive immunological reaction that then protects the body from subsequent infection. Nevertheless, those who had been vaccinated caught colds merely as easily equally those who had not.
Over the adjacent decade, as the techniques for isolating cold viruses were refined, it became clear that in that location were many more rhinoviruses than first predicted. Researchers realised it would not be possible to make a vaccine in the traditional style. Producing dozens of single-serotype vaccines, each 1 targeting a dissimilar strain, would exist impractical. The consensus that a rhinovirus vaccine was not possible deepened. The final homo clinical trial took identify in 1975.
Then, in Jan terminal twelvemonth, an editorial appeared in the Practiced Review of Vaccines that once again raised the prospect of a vaccine. The commodity was co-authored by a group of the world's leading respiratory affliction specialists based at Majestic College London. It was worded cautiously, even so the claim it fabricated was striking. "Perhaps the quest for an RV [rhinovirus] vaccine has been dismissed as as well difficult or even impossible," information technology said, "but new developments propose that it may be viable to generate a significant breadth of immune protection." The scientists were claiming to be on the way to solving a riddle that has stumped virologists for decades. One virologist told me it was equally if a door that had been closed for many, many years had been re-opened.
Part of the Purple scientists' motivation was the notion that since we at present take vaccines for many of the most dangerous viruses (measles, polio, yellowish fever, cholera, flu, and then on), it is time to tackle the disease that afflicts us well-nigh oftentimes. "Rhinovirus is by far the near common crusade of illness," says Sebastian Johnston, a professor at Regal and i of the authors of the editorial. "Look at what people spend on ineffective over-the-counter medications. If you had a safety and constructive treatment, yous'd take information technology."
I asked Johnston if he was optimistic. He pointed out that considering their studies so far have only been in mice, they are non certain that the vaccine will work in humans. "The data is limited," he says. "But it's encouraging." It was not the resounding triumphalism that I was expecting, but then cold scientists learned long ago to exist careful about making grand proclamations. Theirs is an undertaking that, more than annihilation, has been defined by consistent thwarting.
T he showtime scientist to endeavor and fail to make a rhinovirus vaccine was as well the first scientist to distinguish it from the jumble of other cold viruses. In 1953, an epidemiologist chosen Winston Price was working at Johns Hopkins University in Baltimore when a grouping of nurses in his department came downward with a mild fever, a cough, sore throat and runny olfactory organ – symptoms that suggested the flu. Price took nasal washings from the nurses and grew their virus in a jail cell civilisation. What he found was besides pocket-size to exist influenza virus. In a 1957 paper, "The isolation of a new virus associated with respiratory clinical disease in humans", Cost initially named his discovery "JH virus", after his employer.
Cost decided to attempt to develop a vaccine using a bit of expressionless rhinovirus. When the immune system encounters an invading virus – even a expressionless or weakened virus – it sets out to expel it. One defence force is the production of antibodies, small proteins that hang effectually in the claret system long subsequently the virus is gone. If the virus is encountered a 2d time, the antibodies will swiftly recognise it and raise the alert, giving the allowed system the upper manus.
At showtime, Price was encouraged. In a trial that involved several hundred people, those vaccinated with JH virus had 8 times fewer colds than the unvaccinated. Newspapers across the U.s. wanted to know: had the mutual common cold been cured? "The phone by my bed kept ringing until three o'clock in the morning," Price told the New York Times in November 1957. The celebration would be short-lived. Though Cost'southward vaccine was constructive against his particular "JH" rhinovirus strain, in subsequent experiments information technology did nothing. This indicated that more one rhinovirus was out there.
By the tardily 1960s, dozens of rhinoviruses had been discovered. Fifty-fifty in the conflicting menagerie of respiratory affliction, this level of variation in one species was unusual; at that place are merely three or four influenza viruses circulating at any one fourth dimension. Scientists at the University of Virginia decided to try a different tactic. Instead of inoculating patients with a single strain of rhinovirus, they combined 10 different serotypes in one injection. But after this, too, failed to shield participants from infection, they were out of ideas.
As hope for a vaccine receded, scientists began investigating other means to gainsay colds. From 1946 until information technology closed in 1990, most inquiry into respiratory viruses in the United kingdom of great britain and northern ireland was undertaken at the Common cold Unit of measurement (CCU), a facility backed by the Medical Enquiry Council that occupied a former wartime military hospital in the countryside near Salisbury. In its four decades of operation, some 20,000 volunteers passed through the doors of the CCU, many to be willingly infected with cold virus in the proper name of scientific progress.
An early experiment at the CCU involved a grouping of volunteers existence made to accept a bathroom and then to stand up dripping wet and shivering in a corridor for 30 minutes. After they were allowed to go dressed, they had to vesture moisture socks for several hours. Despite a drib in trunk temperature, the grouping did not get any more colds than a control group of volunteers who had been kept cosy.

The CCU began focusing on cold treatments in the 1960s and 70s, when research into a substance produced by the man torso chosen interferon was gaining momentum. Interferons are proteins that are secreted by cells when they are attacked by a virus. They human action as messengers, alerting nearby cells to the invader. These cells in turn produce an antiviral protein that inhibits, or interferes with, the virus'due south ability to spread, hence the name.
In 1972, researchers at the CCU decided to investigate whether interferon could be used equally a handling for colds. They infected 32 volunteers with rhinovirus and then sprayed either interferon or placebo upwards their noses. Of the sixteen given a placebo, 13 came down with colds. Simply of the 16 given interferon, only three got ill. The findings, published in The Lancet, made the front folio of the New York Times (below a story on Watergate). A rush of interferon research got underway. But, once again, the excitement was premature. A review by the CCU in the 1980s uncovered a fatal flaw: interferon only worked when information technology was given to the patient at the same time equally the virus. But in real life – that is, exterior the lab – a rhinovirus enters the nose between eight and 48 hours before the onset of cold symptoms. Past the time you feel a cold coming on, it is already too late.
Equally the 20th century drew to a close, attempts to detect a cure grew more than drastic. At the CCU, molecules that were institute in traditional Chinese medicine, Japanese tea and oranges were all seriously interrogated. In 1990, the CCU closed. The eye had done much to advance our understanding of the virology of the cold, yet it had besides exposed the enormity of the task of defeating information technology.
In the 1990s, as many virologists focused on HIV and Aids, research into the cold tailed off. "Common astute respiratory infections were seen as less of import compared with this threat of a worldwide, lethal plague," writes David Tyrrell, the sometime managing director of the CCU, in his 2002 book Cold Wars. A cure seemed more remote than always.
S ebastian Johnston's lab is on the third floor of the School of Medicine, part of Majestic College'southward St Mary's Infirmary campus in Paddington, west London. Opened in 1851, the original hospital building is red-brick, with high ceilings, arched colonnades and turrets, only numerous extensions, each progressively more than box-like, now hem it in. A round blue plaque on the facade states that Sir Alexander Fleming (1881-1955) discovered penicillin in a second-storey room. Entry to a recreation of Fleming'due south lab is £iv.
Johnston, a professor of respiratory medicine and an asthma specialist, is 58 and bespectacled, with a mop of gray curls that form a height on his forehead. Equally a PhD educatee in 1989, he was dispatched to the CCU, not long before it closed down, to study virus detection methods. "I spent six months at that place," Johnston said. "It was a strange place, basically a bunch of nissen huts continued by wooden runways, with lots of rabbits."
For his PhD on asthma, Johnston developed a technique called polymerase chain reaction, which magnifies DNA so that viruses can be identified more than precisely. To his anaesthesia, Johnston discovered that viruses were backside 85% of asthma attacks in children; about half of those were rhinoviruses. Previously, most studies had detected viruses in fewer than 20% of asthma attacks. Johnston went on to find that rhinovirus also exacerbates symptoms in 95% of cases of smoker'due south cough (formally known as chronic obstructive pulmonary disease, or COPD).
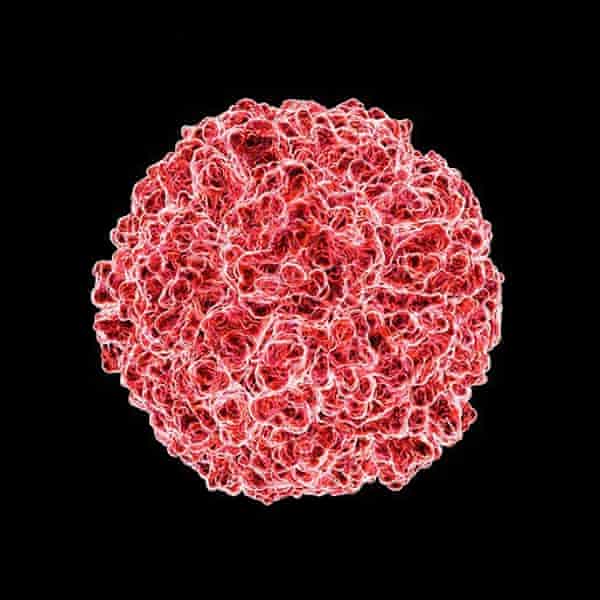
Information technology wasn't until the 1990s that scientists fighting rhinovirus properly understood what they were up against. By that time, electron microscopy had advanced and it was possible to see the organism up close. For a pathogen so spectacularly adept at infecting our nasal passages – the "rhin" of the name is from the Greek for "nose" – rhinoviruses are astonishingly simple, being fiddling more than strands of ribonucleic acid (RNA) surrounded by a beat: "a piece of bad news wrapped in a protein coat", every bit the Nobel Prize-winning biologist Peter Medawar once observed. Under an electron microscope, they are spherical with a shaggy surface like the bobble on a knitted chapeau.
Though all the rhinoviruses are pretty much the aforementioned internally, a subtle alteration to the pattern of proteins on their outer trounce means that, to the allowed system, they all look dissimilar. It's a cloak-and-dagger strategy, and the reason why early vaccines such as Winston Price'southward failed. Antibodies produced for one rhinovirus serotype do not detect the rest. Until recently, it was believed that there were effectually 100 different strains, and these were grouped into the "A" and "B" families. So, in 2007, a new cache of viruses was discovered, the "C" grouping, making the full more than like 160.
In 2003, Johnston, who was then working at Imperial, contacted Jeffrey Almond, a former professor of virology at Reading University who had been recently appointed as head of vaccine development at the pharmaceutical giant Sanofi. The company was already manufacturing a jab for flu and was interested in tackling the common cold. Having bumped into Johnston at academic conferences, Almond felt that their ambitions were aligned. "I said: 'Allow's think well-nigh whether we can do something dramatic,'" Almond told me. "Permit'due south think nigh how we can make a vaccine against rhino."
For doctors, vaccines are preferable to drugs considering they shield the host from invasive organisms before they cause any damage. For pharmaceutical companies, vaccines are significantly less attractive. Not only practice they take years and hundreds of millions of dollars to develop, fifty-fifty if that procedure is successful – which it frequently isn't – it tin notwithstanding exist hard to brand much money. Vaccines are normally injections administered on a unmarried occasion, while drugs are taken for prolonged periods. And people don't want to pay much for vaccines. "Everybody wants vaccines for pennies rather than pounds because you lot get them when you're healthy," Almond said. "Nobody wants to pay anything when they're good for you. It's like automobile insurance, correct? But when y'all're sick you will empty your wallet, whatever information technology takes."
However, Almond thought there might be a commercial case for a rhinovirus vaccine. Totting up the days off schoolhouse and work, plus the secondary infections such as sinusitis that require supplementary treatment and even hospitalisation, rhinovirus places a huge burden on wellness systems. Final year, in the U.k., coughs and colds accounted for near a quarter of the total number of days lost to sickness, well-nigh 34m. In the US, a survey carried out in 2002 calculated that each common cold experienced past an adult causes an average loss of 8.7 working hours, while a farther 1.2 hours are lost attending to cold-ridden children, making the total cost of lost productivity most $25bn (£19bn) each year. Almond convinced his bosses that, if it were possible to make i, a rhinovirus vaccination would be financially viable. "Our back-of-the-envelope calculations on what we could charge, and what the numbers of sales could be, mean that information technology's likely to exist quite profitable and quite interesting for a company to develop," Almond says.
Reviewing the approaches taken in the 1960s and 70s, Almond and Johnston dismissed the idea of a mega-vaccine of all the 160 rhinovirus serotypes, believing it would be too heavy, too complex and likewise expensive to brand. They wondered instead if there was a tiny part of the structure of viruses that is identical, or "conserved", across the entire species that could class the footing of what is called a subunit vaccine, an arroyo that has had success with hepatitis B and the homo papilloma virus, or HPV.
After comparison the genetic sequences of the different rhinovirus serotypes, the researchers honed in on a particular protein on the virus shell that seemed to recur beyond many of the serotypes. They took a piece of the conserved shell from a single rhinovirus, number 16, and mixed it with an adjuvant – a stimulus that mimics the danger signals that trigger an allowed response – and injected information technology into mice as a vaccine. The promise was that the allowed organization would exist jolted into recognising the beat out protein as an invasive pathogen, conferring immunity against the entire rhinovirus family unit.
In petri dishes, the scientists mixed the immunised mouse blood with three other rhinovirus serotypes, numbers ane, 14 and 29. An immunological response to rhinovirus ane was probable because its genetic sequence is similar to sixteen, simply serotypes 14 and 29 are unalike. The mice's white claret cells responded vigorously confronting all three strains. "Seeing responses against those ii [different serotypes] was very encouraging," Johnston said. This gave promise that the vaccine might protect confronting the full gamut of rhinoviruses.
The scientists gathered a group of respiratory medicine specialists to review the findings. The reviewers agreed that the results looked promising. But merely as the scientists were prepare to take the vaccine forward, there was a setback at Sanofi. "There was a modify of management, a change of guys at the top," Almond said. "I took early retirement for different reasons. My boss retired equally well."
In 2013, the new management decided that the company's priorities were elsewhere, handing dorsum to Imperial College the patent that protects the vaccine idea from existence developed past other groups. Regal did non accept the resource to develop the vaccine without outside investment. For Johnston, information technology was frustrating – years of research and toil in the lab had seemed to be finally yielding results. Merely there was little he could do. The vaccine was shelved.
A cross the Atlantic, equally Purple began to search for new backers, Martin Moore, a paediatrician at Emory University in Atlanta, was working on a rival approach to the same problem. A specialist in children's respiratory disease, for the by three years Moore has been working on a solution so straightforward that when he presented the results of his newspaper, published in Nature Communications concluding twelvemonth, his colleagues struggled to take them. "Just if I pushed them, I couldn't get a good reason for that other than, just: it hadn't been done before," he says.
Moore first resolved to do something about the common cold in 2014, while on holiday with his family unit in Florida. Before long later on they had arrived, his son, then a toddler, came downwardly with a cold. "He wanted me to hold him twenty-four hour period and night," Moore said. The pair hunkered downward in the hotel room watching movies while the remainder of the family unit went to the beach. "It was frustrating because, as a virologist, we can go into the lab and slice and dice these viruses. But what are we actually doing about them?"
Moore reviewed the papers from the 1960s and 70s that described the early attempts at a vaccine. He saw that the scientists had demonstrated that if they took one rhinovirus, killed it and then injected it, it would protect people against that same strain. "People actually made decent vaccines against rhinovirus in the 1960s," Moore told me. What scientists did not business relationship for at the time was that there were so many different serotypes. Just where the scientists of the past had seen defeat, Moore saw promise. Why non only make a vaccine made up of all the rhinoviruses? There was nothing to suggest that information technology would non work. The problem was non with the science, simply with logistics. "I thought, the merely matter between u.s. and doing this is manufacturing and economic science."
Moore secured funding from the National Institutes of Health (NIH) and practical for samples of the unlike serotypes from the Centers for Illness Control and the American Type Civilisation Collection, a biological material repository headquartered in Virginia. He stopped short of calling in all 160 serotypes, reasoning that 50 would exist enough to support his hypothesis.
After developing the vaccine, composed of these 50 serotypes, Moore tested it on a number of rhesus macaque monkeys. When their blood was after mixed with viruses in petri dishes, there was a stiff antibiotic response to 49 of the 50 serotypes. It was not possible to see whether the vaccinated monkeys themselves would be protected from colds, since human rhinoviruses practise non infect monkeys. But the ability to induce antibodies in monkey blood does correlate with protection in people.
"Maybe I shouldn't say this, but I never had a doubt that it would produce antibodies," Moore told me. "Our paper was almost showing it can exist washed." There is still a long way to get before Moore'south dream becomes reality. For the vaccine to be tested in a clinical trial, information technology will need to be made under good manufacturing practice (GMP) atmospheric condition – regulations that companies must adhere to for licensing. Nether these regulations, substances need to be kept separate to avert cantankerous-contamination – a substantial challenge for a vaccine that potentially encompasses 160 serotypes (currently, the largest number of serotypes in a single vaccine, for pneumonia, is 23).
For a manufacturing model, Moore is looking to the polio vaccine, since polio and rhinovirus are biologically related. The scale of product would be many times greater, but the basic processes would be alike. In May, Moore'due south showtime-upwards, Meissa Vaccines, received a $225,000 (£170,000) grant from the NIH for work on rhinovirus. He is taking leave from academia to work on the vaccines.
A t this betoken in time, perchance the biggest bulwark to us curing the common cold is commercial. Researchers at universities tin can merely go so far; the near generous grants from bodies such as the U.k. Medical Research Council are around £2m. Information technology falls to pharmaceutical companies to bear out evolution beyond the initial proof of concept. "You're looking at ten-15 years' work, minimum, with teams of people, and you're going to spend $1bn (£760m) at to the lowest degree," Almond told me.
Successes have been rare, and there have been spectacular flops. Final year, shares in United states firm Novavax savage by 83% after its vaccine for RSV, 1 of the virus families responsible for colds, failed in a late-stage clinical trial. While information technology is less common than rhinovirus, RSV can cause great harm and fifty-fifty decease in those with weakened immunity, including infants and the elderly. An constructive vaccine presented an estimated $1bn opportunity for Novavax in the U.s.a. solitary. Before the results came through, principal executive Stanley Erck said it could be "the largest-selling vaccine in the history of vaccines". Just in the phase III trial of elderly patients, information technology did little to protect against infection. In the hours afterwards the news broke, Novavax share prices roughshod from $8.34 to $i.twoscore.
Episodes such equally this have made pharmaceutical companies wary. Today, vaccines constitute less than 5% of the overall pharmaceutical market place, and development is consolidated in a handful of companies: Sanofi Pasteur, GlaxoSmithKline, Pfizer, AstraZeneca, Merck and Johnson & Johnson, among a few other smaller players.
After the $1bn or so spent on development, at that place are also manufacturing and distribution costs to consider. At that place needs to exist a return on the initial investment. "Y'all sure equally hell can't exercise it if there's not a market at the end, you're wasting the visitor'south money, and if yous practise that also often, you'll broke the company," Almond says. "There isn't a conspiracy out at that place that says, 'Let's not do vaccines and so people can get ill and we charge them a lot', aught like that. It genuinely isn't easy."
In August, I called Sebastian Johnston to run into if at that place was any news on his vaccine. He told me that he had merely received confirmation of further funding from Apollo Therapeutics, a startup backed past AstraZeneca, GSK and Johnson & Johnson. This would allow his lab to test the vaccine on more strains of rhinovirus. Johnston believes that if the vaccine proves to be protective confronting, say, xx serotypes, there is a good run a risk information technology will protect confronting all the rhinoviruses. Beginning in October, the research should have about a year and a half. "At that point, I think nosotros'll exist at a stage where nosotros'll be able to become to major vaccine companies."
If the vaccine were to make it through the clinical trials, and was approved by regulators, it would first be rolled out to high-risk groups – those with asthma and COPD, and possibly the elderly, as the flu jab is in the United kingdom – and then to the rest of the population. In time, every bit the proportion of vaccinated individuals achieve a critical mass, the viruses would cease to circulate because the chain of infection will be broken – a phenomenon chosen herd amnesty.
From where nosotros are today, this scenario is still distant: near 80% of drugs that get in into clinical trials because they worked in mice exercise non go on to piece of work in humans. Nevertheless, for the starting time time in decades in that location are now major pharmaceutical companies with rhinovirus vaccine programmes, as well equally smaller academy research groups like Johnston's which, through different approaches, are all pursuing the same goal of a cure. Once more, Johnston said, "people are starting to believe it may be possible."
Illustrations past Nathalie Lees
Follow the Long Read on Twitter at @gdnlongread, or sign upward to the long read weekly e-mail here.
Source: https://www.theguardian.com/news/2017/oct/06/why-cant-we-cure-the-common-cold
0 Response to "When Is It Going to Get Cold Again 2017"
Post a Comment